
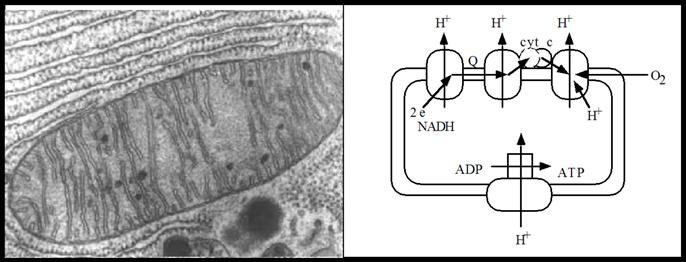
One of the most interesting biochemical systems where coupled ET and PT reactions play the role is
cytochrome c oxidase (CcO). This membrane-bound protein is the terminal enzyme of the cell
respiratory electron-transport chain
in mitochondria and aerobic bacteria.
The energy stored by the gradient subsequently drives synthesis of adenosine triphosphate (ATP),
the key molecule in intracellular energy transfer involved in converting food into energy.
Mitochondrion - Powerhouse of the cell
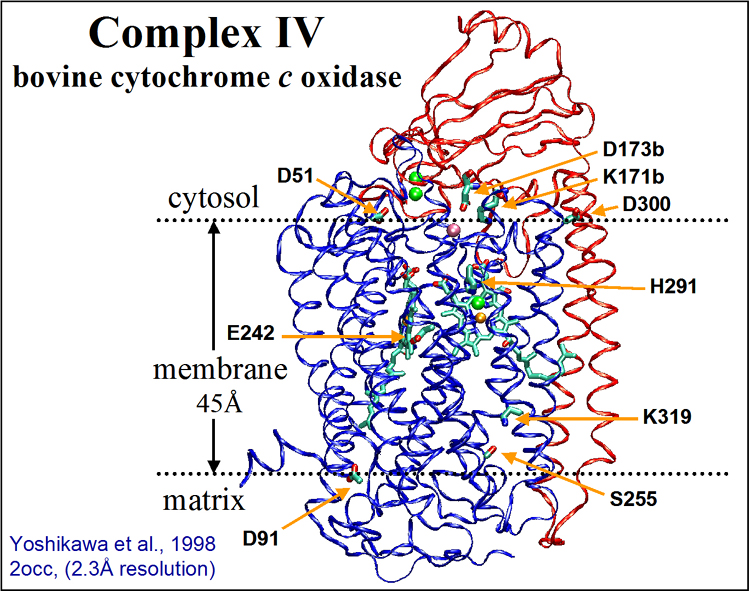
The crystal structure of CcO has been solved for several organisms, but the mechanism of how electrons
and protons are coupled in the enzyme is still an ongoing unsolved problem. In our work of past few years,
we proposed the mechanism of proton pumping of this enzyme, which is presented here.
CcO Project
CcO uses electrons and protons to catalyse the reduction of O2 to H2O and utilize the free energy of
the reduction reaction for a proton pumping across the inner-mitochondrial membrane, a process which
results in a generation of a membrane electrochemical proton gradient.
![]()
Electrostatic Potential of CcO
Water in Catalytic Site
Proposed Proton Pumping Mechanism
Proton Exit Pathway
Conformational Changes of Glu242
Improved DFT/Electrostatic pKa Calculations
Conclusions