Proton Exit Pathway in
Cytochrome c Oxidase
Possible Proton Exit Channels in Bovine CcO
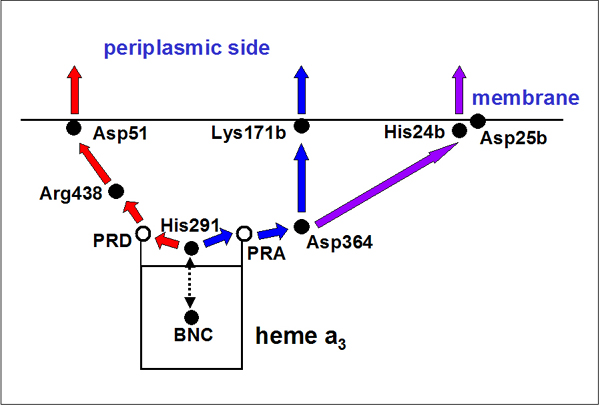
Lys171B/Asp173B Proton Exit Channel
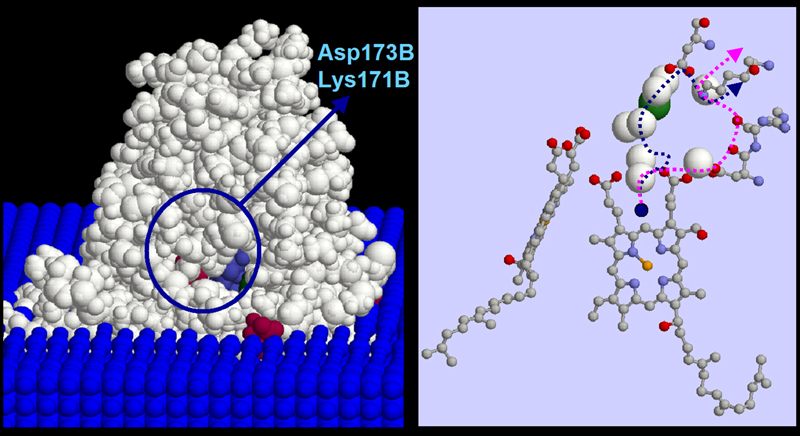
Popovic & Stuchebrukhov, JPC B 109, 1999 (2005)
Based on energetics of the possible proton exit pathways, the most likely proton exit channel
leads to Lys171B/Asp173B site.
A numerous of water molecules, located above the heme propionates, may provide the pathway for
a proton translocation from His291 to Lys171B exit point (via Mg-center, shown as green sphere). Alternatively, a proton
might possibly move along the O atoms of COO– and backbone carbonyl groups.
A very recent mutagenic studies, from Gennis (Lys171B) and Brzezinski (Thr339A) groups, seem to agree
very well with our prediction of proton exit pathway.
Energetics of the Proton Exit Channel (eV)
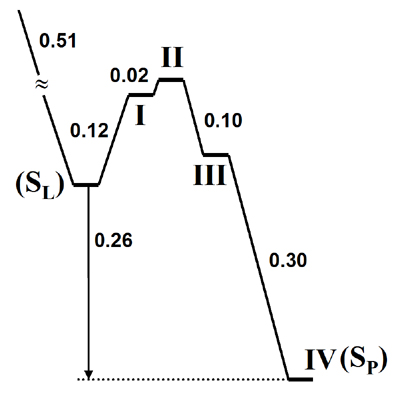
We calculated the energy diagram of multi-step proton transfer reactions along the potential exit channels and the energy diagram is shown here. SL is a metastable state with 2 repulsively interacting protons in active site (one on PLS and other on the BNC). SP is a final state after expulsion of the pump proton from the system. The energy barrier for a back stroke is over 0.4 eV and might be high enough to prevent a proton leaking into pump.
Coulomb Proton Pump with Kinetic Gating (Proton Machine Gun)
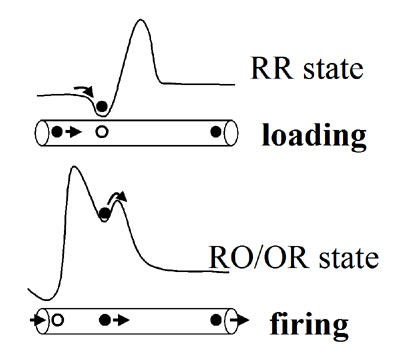
The basic physical principle, which we believe this enzyme is utilize in its pumping mechanism, is summarized here.
There are 2 stages: loading and firing of the pump proton.
Loading stage occurs in RR redox state of the binuclear center (BNC), when the PLS (His291) gets protonated.
The firing stage occurs after a chemical proton enters the active site in RO (or OR) state of the BNC.
Due to the repulsion between the two protons – pump proton get ejected, energetically stabilizing the system.
The switch between the two profiles occurs after an electron transfer from heme a to the BNC takes place.