Computational Methods
I. Continuum electrostatic method
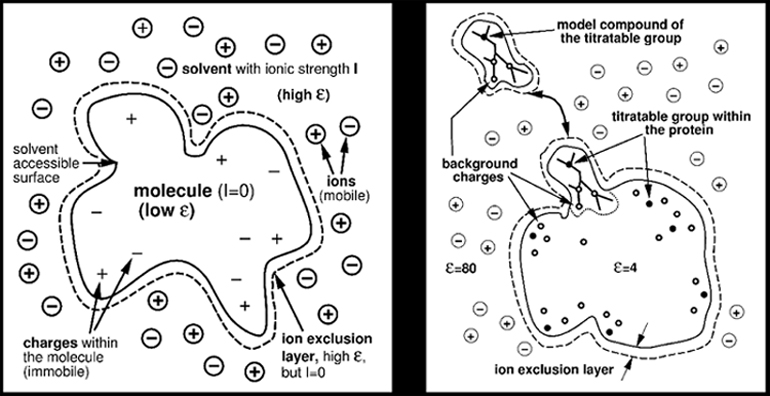
The model of protein in solution (left)
A titratable group in a protein and the corresponding model compound in ionic aqueous solution (right)
Classical continuum electrostatic calculations consist of two parts:
1) Solving the Poisson-Boltzmann equation on a grid:
2) Titration of protein:
where energy of any particular protonation/redox state n of protein defined by state vector xn is :
Some detail of classical electrostatic calculations applied on CcO:
● electrostatic potential for protein-membrane-solvent system was solved by the PB eq. on a grid,
using a finite difference method [MULTIFLEX];
II. DFT/electrostatic (solvation energy) calculations
To calculate pKa's of the binuclear center in CcO, we applied the combined DFT/electrostatic method.
Method itself is a stepwise procedure, where the active site of the enzyme is treated
with the high level of quantum-mechanical calculations.
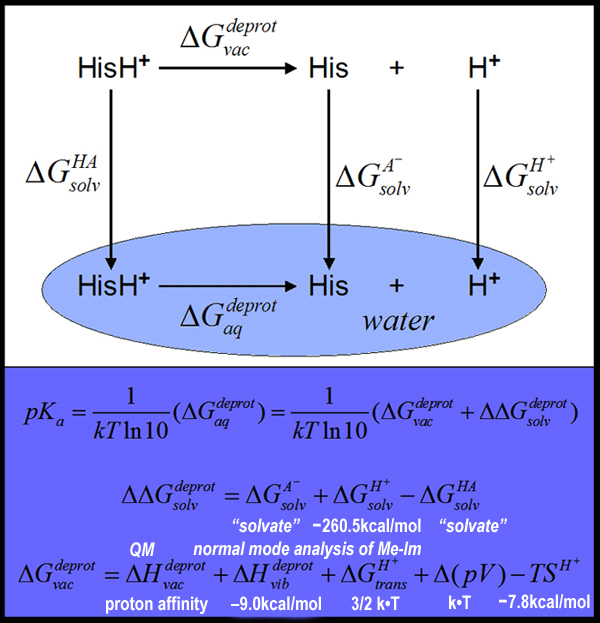
a) The absolute pKa calculations
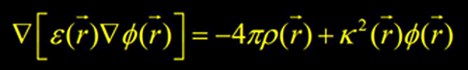
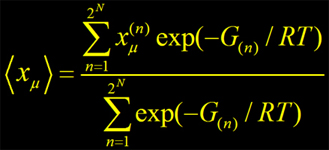 - protonation/oxidation probability
- protonation/oxidation probability
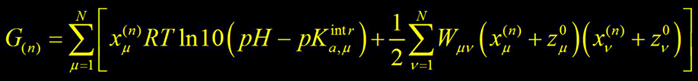
● protonation states of titratable sites were sampled by a Monte-Carlo titration technics;
● CHARMM22 partial atomic charges and radii were used for standard amino-acid residues,
but quantum-chemically calculated atomic charges for non-standard residues and all metal centers;
● x-ray structure of the mitochondrial bovine heart– 2OCC (Yoshikawa,'98) at 2.3Å resolution is used;
● investigated system consists of two subunits – A and B embedded in the membrane slab of 45Å;
● in separate set of computations H2O/OH– ligand of CuB was also considered;
● to explore the energetics of the ET between the hemes, an electron was allowed to equilibrate
between two hemes in MC moves during the sampling procedure;
● energetics of the coupled proton and electron transfer to the binuclear center was evaluated.
The rest of the protein is treated electrostatically as the protein point charges,
including the dielectric inhomogenities, inner water filled cavities, external solvent and membrane.
All of them together produce the electric field, so called reaction and protein field, which act on the
QM system itself and shift energy of the protonation reaction in the BNC.

b) The relative pKa calculations
A stepwise procedure:
1) Standard DFT calc. => to optimize the structures of the complex in vacuum and to obtain electronic gas phase energies for different redox/protonation states of the complex.
2) DFT-SCRF calc. => ESP set of atomic point charges for the active site complex in corresponding dielectric environments and reorganization energies.
3) Classical electrostatic calc. => equilibrium proton (charge) distribution in CcO for the corresponding redox state of metal centers.
4) Solvation electrostatic calc. => active site complex is docked back into protein and reaction and protein field terms are calculated.
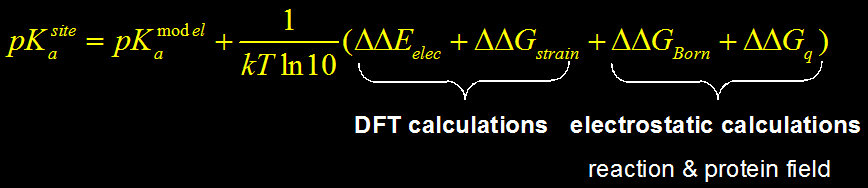
5) Different energy terms are combined to calculate the pKa: