Electron Tunneling in Complex I
|
Specific peptide residues serve as electronic
wires connecting neighboring Fe/S clusters; individual electron
tunneling paths involve up to three protein residues, including
two cysteine ligands and one additional key residue. The clusters in the protein are oriented in a specific way - corner to corner - with Cys ligands mostly pointing toward each other, which is clearly the most efficient way to transfer electrons from one cluster to another. |
|
|
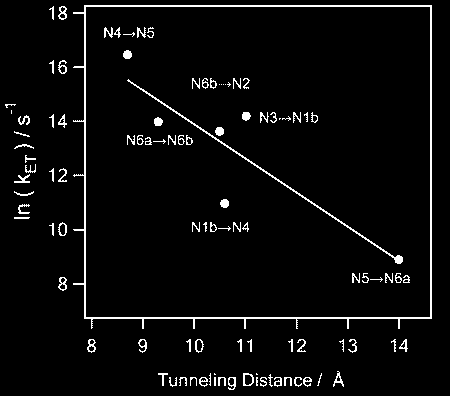
The internal water between the protein subunits has been found an essential mediator for the efficient electron transfer along the redox chain of complex I by
accelerating the electron transfer kinetics so as to achieve the physiologically meaningful rate.
With the water included, the overall negative slope of the distance dependence of the electron transfer rates in natural logarithm becomes close to a typical 1.4. (See our paper for details) |